
UHN REMEDI PET/MR & Cyclotron Radiopharmacy Facility
Toronto General Hospital 200 Elizabeth Street, Toronto, ON
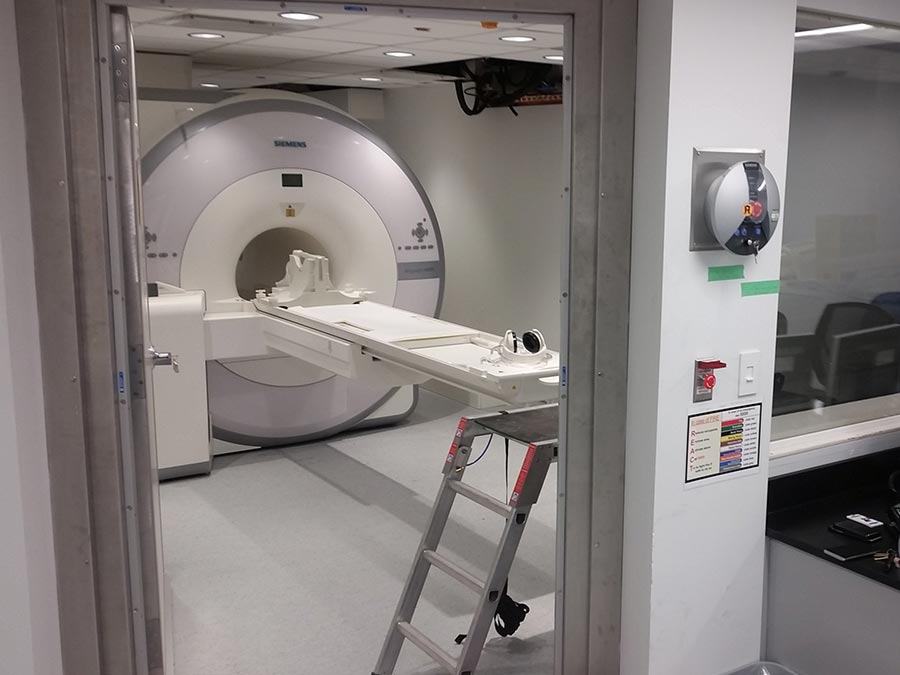
The PET/MR and Cyclotron facilities we installed at the Toronto General Hospital are one of the first imaging facilities of its kind in North America.
Scope of Work:
Phase 1: Cyclotron facility construction and installation.
Phase 2: Construction of radiopharmacy facilities.
Phase 3: Construction of attached PET/MR facilities.
- Renovation of approximately 9,800 square feet of existing vacant and un-serviced space of a hospital wing on the basement level of the Clinical Services building.
- Renovation into a first-class radiopharmaceutical isotope production facility, including a combined cyclotron and PET/MR facility.
- Three-phase construction project completed without disturbing normal hospital operations.
- Heavy concrete and lead shielding requirements due to cyclotron and PET/MR radiation emissions.
- Interior programming, fit-out and construction of a 4,500 square foot integrated cyclotron-radiopharmacy facility for advanced patient and animal testing/research and cancer treatments.
- Creation of a one-of-a-kind facility for production of radiochemically pure isotopes for use in various modes of imaging.
- Support spaces included the cyclotron equipment room, mechanical and electrical support spaces, storage rooms, gas farm, ante room, QC room storage, quarantine, research and development, generator room, WBC room, radioactive waste room, vivarial, radiopharmacy, and office space.
- Major rework to existing facilities and infrastructure to accommodate large equipment into the space without disturbing surrounding hospital area.




 @IraMcdonald
@IraMcdonald
 LinkedIn
LinkedIn